

2025 , Vol. 22 >Issue 03: 203 - 208
DOI: https://doi.org/10.3877/cma.j.issn.1672-6448.2025.03.003
出生后不同狭窄程度及是否手术干预的孤立性肺动脉狭窄胎儿产前超声图像特征
Copy editor: 吴春凤
收稿日期: 2024-12-19
网络出版日期: 2025-06-10
版权
Prenatal ultrasound characteristics of isolated pulmonary stenosis in fetuses according to postnatal stenosis severity and surgical intervention
Received date: 2024-12-19
Online published: 2025-06-10
Copyright
目的
探讨出生后不同狭窄程度及是否手术干预的孤立性肺动脉狭窄(IPS)胎儿的产前超声图像特征。
方法
回顾性分析2018年4月至2023年4月安徽医科大学第一附属医院、安徽省妇幼保健院、安徽医科大学第二附属医院3家产前诊断中心确诊的59例IPS胎儿的临床资料及产前超声资料。根据出生后1周内超声心动图结果,将病例分为中-重度肺动脉狭窄组和轻度肺动脉狭窄组;根据是否行手术治疗分为手术组和非手术组。采用单因素分析(Fisher精确检验、t检验或Mann-Whitney U检验)比较组间产前超声参数的差异。
结果
59例产前诊断IPS病例中,18例选择终止妊娠,41例继续妊娠至分娩且胎儿均存活。与轻度肺动脉狭窄组(n=32)比较,中-重度肺动脉狭窄组(n=9)产前超声提示肺动脉瓣内径/肺动脉主干内径比值<0.5、右心异常及存在异常指标数≥3个患儿比例更高[67% vs 22%;67% vs 6%;78% vs 19%],卵圆孔内径更大[6.40(4.90,8.05)mm vs 4.90(4.43,5.30)mm],差异具有统计学意义(P=0.018;P<0.001;P=0.002;Z=-2.175,P=0.029)。与非手术组(n=33)比较,手术组(n=8)产前超声提示存在肺动脉瓣环内径/肺动脉主干内径比值<0.5、存在肺动脉狭窄后扩张、三尖瓣反流流速>100 cm/s、右心异常、异常指标数目≥3个患儿比例更高[75% vs 21%;87% vs 36%;62% vs 21%;75% vs 6%;75% vs 21%],卵圆孔内径更大[6.40(4.90,8.05)mm vs 4.90(4.43,5.30)mm],差异具有统计学意义(P=0.007;P=0.016;P=0.034;P<0.001;P=0.007;Z=-2.881,P=0.003)。
结论
中-重度肺动脉狭窄及进行手术干预的IPS胎儿肺动脉瓣环内径/肺动脉主干内径比值、右心异常、卵圆孔内径及异常参数数目≥3个等指标的患儿比例或数值更高。
孙慧洁 , 冯新嫄 , 刘天赐 , 刘彦昭 , 锁仁静 , 罗平 , 李亮 . 出生后不同狭窄程度及是否手术干预的孤立性肺动脉狭窄胎儿产前超声图像特征[J]. 中华医学超声杂志(电子版), 2025 , 22(03) : 203 -208 . DOI: 10.3877/cma.j.issn.1672-6448.2025.03.003
Objective
To investigate the prenatal ultrasound characteristics of isolated pulmonary stenosis (IPS) in fetuses associated with postnatal stenosis severity and surgical intervention outcomes.
Methods
A retrospective analysis was conducted on 59 cases of fetal IPS diagnosed from April 2018 to April 2023 at three prenatal diagnosis centers: the First Affiliated Hospital of Anhui Medical University, Anhui Maternal and Child Health Hospital, and the Second Affiliated Hospital of Anhui Medical University. Clinical data and prenatal ultrasound findings were collected and analyzed. Based on echocardiographic findings within the first week after birth, the cases were categorized into two groups: moderate-to-severe pulmonary stenosis and mild pulmonary stenosis. Additionally, the cases were further stratified into surgical and non-surgical groups based on whether they underwent surgical intervention. Differences in prenatal ultrasound parameters between groups were compared using univariate analysis, including Fisher’s exact test, Student’s t-test, or Mann-Whitney U test, as appropriate.
Results
Among the 59 prenatally diagnosed cases, 18 opted for pregnancy termination, while 41 continued the pregnancy to delivery, with all fetuses surviving. Compared with the mild pulmonary valve stenosis group (n=32), the moderate-to-severe pulmonary valve stenosis group(n=9) had a higher proportion of patients with a pulmonary valve diameter/main pulmonary artery diameter ratio <0.5 on prenatal ultrasound, right ventricular abnormalities, and ≥3 abnormal indicators [67% vs 22%;67% vs 6%; 78% vs 19%], as well as a larger foramen ovale diameter [6.40 (4.90, 8.05) mm vs 4.90 (4.43, 5.30)mm]; these differences were statistically significant (P=0.018, P<0.001, P=0.002, Z=-2.175, and P=0.029,respectively). Compared with the non-surgical group (n=33), the surgical group (n=8) had a higher proportion of patients with a pulmonary valve annulus diameter/main pulmonary artery diameter ratio <0.5, post-stenotic dilation of the pulmonary artery, tricuspid regurgitation velocity >100 cm/s, right ventricular abnormalities,and ≥3 abnormal indicators [75% vs 21%; 87% vs 36%; 62% vs 21%; 75% vs 6%; 75% vs 21%], as well as a larger foramen ovale diameter; these differences were statistically significant (P=0.007, P=0.016, P=0.034,P<0.001, P=0.007, Z=-2.881, and P=0.003, respectively).
Conclusion
In fetuses with moderate-to-severe pulmonary valve stenosis and those requiring surgical intervention, the ratio of pulmonary valve annulus diameter to main pulmonary artery diameter, the incidence of right ventricular abnormalities, the size of the foramen ovale, and the number of abnormal parameters ≥3 are higher.
Key words: Isolated pulmonary stenosis; Fetus; Echocardiogram; Prognosis
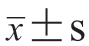 表示,采用独立样本t检验比较组间差异,肺动脉瓣环内径、肺动脉瓣处血液流速、肺动脉主干内径/主动脉主干内径比值、卵圆孔内径为非正态分布的计量资料,以M(Q1,Q3)表示,采用秩和检验比较组间差异。P<0.05为差异具有统计学意义。
表示,采用独立样本t检验比较组间差异,肺动脉瓣环内径、肺动脉瓣处血液流速、肺动脉主干内径/主动脉主干内径比值、卵圆孔内径为非正态分布的计量资料,以M(Q1,Q3)表示,采用秩和检验比较组间差异。P<0.05为差异具有统计学意义。表1 孤立性肺动脉狭窄胎儿中-重度与轻度肺动脉狭窄组及手术组与未手术组孕妇年龄及孕周分布比较(例) |
| 组别 | 例数 | 孕妇年龄 | 孕周 | ||||
|---|---|---|---|---|---|---|---|
| ≥ 35 岁 | <35 岁 | 中孕期 | 晚孕期 | ||||
| 中- 重度肺动脉狭窄组 | 9 | 3 | 6 | 3 | 6 | ||
| 轻度肺动脉狭窄组 | 32 | 3 | 29 | 19 | 13 | ||
| P值 | 0.107 | 0.260 | |||||
| 手术组 | 8 | 3 | 5 | 3 | 5 | ||
| 未手术组 | 33 | 3 | 30 | 19 | 14 | ||
| P值 | 0.077 | 0.436 | |||||
表2 不同肺动脉狭窄程度的孤立性肺动脉狭窄患儿产前超声参数的差异性分析 |
| 变量 | 中- 重度肺动脉狭窄组(n=9) | 轻度肺动脉狭窄组(n=32) | 统计值 | P值 |
|---|---|---|---|---|
| 肺动脉瓣环内径[mm,M ( Q1, Q3 )] | 4.20(3320,4.40) | 4.20(3.15,5.98) | Z=-0.457 | 0.653 |
| 肺动脉瓣处流速[cm/s,M ( Q1, Q3 )] | 134.00(91.50,318.50) | 150.00(121.50,183.75) | Z=-0.252 | 0.816 |
| 肺动脉主干内径(mm,± s ) | 7.53±1.87 | 7.03±1.89 | t=0.033 | 0.488 |
| 肺动脉瓣环内径/ 主干内径比值<0.5[ 例(%)] | 6(67) | 7(22) | - | 0.018 |
| 存在肺动脉瓣关闭不全[ 例(%)] | 3(33) | 7(22) | - | 0.622 |
| 肺动脉主干内径/ 主动脉主干内径比值[M ( Q1, Q3 )] | 1.76(1.31,1.88) | 1.57(1.15,1.75) | Z=-1.575 | 0.120 |
| 存在肺动脉狭窄伴狭窄后扩张[ 例(%)] | 7(78) | 12(38) | - | 0.057 |
| 三尖瓣反流[ 例(%)] | 4(45) | 13(41) | - | 1.000 |
| 三尖瓣反流流速>100 cm/s[ 例(%)] | 5(55) | 7(22) | - | 0.093 |
| 右心异常[ 例(%)] | 6(67) | 2(6) | - | <0.001 |
| 卵圆孔内径[mm,M ( Q1, Q3 )] | 6.40(4.90,8.05) | 4.90(4.43,5.30) | Z=-2.175 | 0.029 |
| 存在异常指标数目≥ 3 个[ 例(%)] | 7(78) | 6(19) | - | 0.002 |
注:(1)右心异常包括右心室壁增厚、右心增大;(2)异常指标包括肺动脉狭窄、肺动脉流速>1.4 m/s、肺动脉关闭不全、肺动脉狭窄伴狭窄后扩张、三尖瓣反流、三尖瓣反流最大流速>1 m/s、卵圆孔增大、右心异常、动脉导管逆灌、肺动脉瓣环内径/肺动脉主干内径比值<0.5。-表示采用Fisher确切检验,无相应统计值 |
表3 孤立性肺动脉狭窄患儿是否手术组产前超声参数的差异性分析 |
| 变量 | 手术组(n=8) | 未手术组(n=33) | 统计值 | P值 |
|---|---|---|---|---|
| 肺动脉瓣环内径[mm,M ( Q1, Q3 )] | [4.15(3.20,4.30)] | [4.20(3.20,5.95)] | Z=-0.741 | 0.467 |
| 肺动脉瓣处流速[cm/s,M ( Q1, Q3 )] | [132.50(87.25,333.25)] | [150.00(122.00,190.00)] | Z=-0.181 | 0.895 |
| 肺动脉主干内径(mm, ± s ) | 7.61±1.98 | 7.03±1.87 | t=0.255 | 0.438 |
| 肺动脉瓣环内径/ 主干内径比值< 0.5[ 例(%)] | 6(75) | 7(21) | - | 0.007 |
| 存在肺动脉瓣关闭不全[ 例(%)] | 2(25) | 8(24) | - | 1.000 |
| 肺动脉主干内径/ 主动脉主干内径比值[M ( Q1, Q3 )] | [1.73(1.25,1.87)] | [1.58(1.17,1.76)] | Z=-0.987 | 0.339 |
| 存在肺动脉狭窄伴狭窄后扩张[ 例(%)] | 8(87) | 12(36) | - | 0.016 |
| 三尖瓣反流[ 例(%)] | 4(50) | 13(39) | - | 0.698 |
| 三尖瓣反流流速> 100 cm/s[ 例(%)] | 5(62) | 7(21) | - | 0.034 |
| 右心异常[ 例(%)] | 6(75) | 2(6) | - | < 0.001 |
| 卵圆孔内径[mm,M ( Q1, Q3 )] | 6.55(5.23,8.38) | 4.90(4.35,5.30) | Z=-2.881 | 0.003 |
| 存在异常指标数目≥ 3 个[ 例(%)] | 6(75) | 7(21) | - | 0.007 |
注:(1)右心异常包括右心室壁增厚、右心增大;(2)异常指标包括肺动脉狭窄、肺动脉流速>1.4 m/s、肺动脉关闭不全、肺动脉狭窄伴窄后扩张、三尖瓣反流、三尖瓣反流最大流速>1 m/s、卵圆孔增大、右心异常、动脉导管逆灌、肺动脉瓣环内径/肺动脉主干内径比值<0.5。-表示采用Fisher确切检验,无相应统计值 |
| 1 |
Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies [J]. Int J Epidemiol, 2019, 48(2): 455-463.
|
| 2 |
Zhao QM, Liu F, Wu L, et al. Prevalence of congenital heart disease at live birth in China [J]. J Pediatr, 2018, 204: 53-58.
|
| 3 |
罗刚, 刘娜, 王葵亮, 等. 婴儿肺动脉瓣狭窄的介入治疗 [J]. 中国实用儿科杂志, 2019, 34(8): 680-684.
|
| 4 |
Mishra S, Lele P. Echocardiography to evaluate pulmonary stenosis [J].J Indian Acad Echocardiogr Cardiovasc Imaging, 2020, 4(3): 276.
|
| 5 |
Marchini F, Meossi S, Passarini G, et al. Pulmonary valve stenosis:from diagnosis to current management techniques and future prospects[J]. Vasc Health Risk Manag, 2023, 19: 379-390.
|
| 6 |
Heaton J, Horenstein MS, Kyriakopoulos C. Pulmonary stenosis[M/OL]. Treasure Island (FL): StatPearls Publishing, 2025 [2024-10-06]. https://www.ncbi.nlm.nih.gov/books/NBK560750/.
|
| 7 |
李博, 彭芳华, 孔德璇, 等. 超声心动图诊断胎儿单纯肺动脉瓣狭窄的价值 [J/OL]. 中华医学超声杂志(电子版), 2024, 21(2): 158-162.
|
| 8 |
Gorla SR, Singh AP. Pulmonary atresia with intact ventricular septum[M/OL]. Treasure Island (FL): StatPearls Publishing, 2025 [2024-07-27]. https://doi.org/10.1007/978-3-031-70973-9_16.
|
| 9 |
孙利华, 马明明, 陈冉, 等. 人工智能在胎儿超声心动图中的应用与进展 [J/OL]. 中华医学超声杂志(电子版), 2024, 21(7): 741-744.
|
| 10 |
Drose JA. Fetal echocardiography [M]. 2nd ed. Philadelphia: Saunders Elsevier, 2009: 169-183.
|
| 11 |
Moon-Grady AJ, Donofrio MT, Gelehrter S, et al. Guidelines and recommendations for performance of the fetal echocardiogram: an update from the American Society of Echocardiography [J]. J Am Soc Echocardiogr, 2023, 36(7): 679-723.
|
| 12 |
马敏亚, 刘浏, 康毛, 等. 产前超声心动图在胎儿肺动脉狭窄诊断中的临床应用价值及产后随访分析 [J]. 哈尔滨医药, 2023, 43(2):32-35.
|
| 13 |
王锟, 张晓花, 赵亚宁, 等. 胎儿超声心动图在单纯肺动脉瓣狭窄分度诊断和预后评估中的应用价值 [J]. 中国超声医学杂志, 2023,39(10): 1142-1145.
|
| 14 |
梁永州, 赵莉晴, 张敏杰, 等. 胎儿肺动脉瓣狭窄的超声心动图特征及生后管理分析 [J]. 中华儿科杂志, 2024, 62(2): 138-144.
|
| 15 |
陈树宝. 先天性心脏病影像诊断学 [M]. 北京: 人民卫生出版社,2004: 246.
|
| 16 |
Hoetama E, Prakoso R, Roebiono PS, et al. Balloon pulmonary valvuloplasty in neonates with critical pulmonary stenosis: jugular or femoral [J]. Ann Pediatr Cardiol, 2020, 13(1): 11-15.
|
| 17 |
Huang KA, Dai X, Liao D, et al. Balloon valvuloplasty via the pulmonary artery trunk for treating neonates with severe pulmonary valve disease [J]. Heart Surg Forum, 2021, 24(1): E185-E187.
|
| 18 |
吕小东, 杨克明, 李守军, 等. 阜外医院71例Rastelli手术的近中期结果 [J]. 中华胸心血管外科杂志, 2020, 36(1): 5-9.
|
| 19 |
张小敏, 赵博文, 潘美, 等. 胎儿心脏定量分析技术对肺动脉狭窄及肺动脉闭锁胎儿心脏形态及功能的初步研究 [J]. 中华超声影像学杂志, 2024, 33(6): 489-496.
|
| 20 |
Cai M, Guo N, Fu M, et al. Prenatal diagnosis of genetic aberrations in fetuses with pulmonary stenosis in southern China: a retrospective analysis [J]. BMC Med Genomics, 2023, 16(1): 119.
|
| 21 |
Dherange P, Telles N, Modi K. Flow-driven right-to-left cardiac shunting in a patient with carcinoid heart disease and patent foramen ovale without elevated right atrial pressure: a case report and literature review [J]. Eur Heart J Case Rep, 2020, 4(6): 1-5.
|
| 22 |
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [J].J Am Coll Cardiol, 2021, 77(4): 450-500.
|
| 23 |
Radu D, Dobrovie M, Capa R, et al. Neurofibromatosis 1-noonan syndrome associated with pulmonary stenosis and hypertrophic cardiomyopathy [J]. Rom J Cardiol, 2021, 31: 903-910.
|
| 24 |
刘一, 石华, 胡佳琪, 等. 早孕期胎儿静脉导管筛查的临床价值 [J].武汉大学学报(医学版), 2020, 41(3): 472-475.
|
/
| 〈 |
|
〉 |