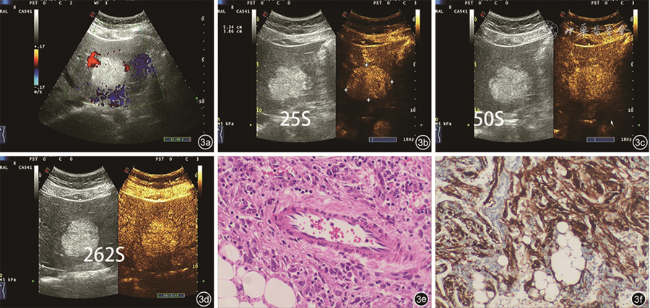
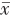病理显示HCA由层状或索状肝细胞及少量Kuffer细胞组成,无汇管区及中心静脉,板层间有扩张的血窦,胆管结构较正常肝组织少,可发生脂肪变性、出血及坏死,血流较丰富
[10],常发生在无乙肝或肝硬化背景的肝脏中
[7,11]。由于HCA复杂的病理变化导致其声像图表现不具有特异性
[12],可表现为高回声、低回声或其他回声;CMAL主要是由平滑肌细胞、成熟的脂肪组织及畸形的厚壁血管按不同比例构成,既往研究报道
[13]二维超声多表现为高回声;而HCC(AFP-)中细胞成分简单、反射界面少而以低回声为主,该表现与本研究的结果相符。大量文献指出
[14, 15],典型的HCA血流信号以周边为主,血管密度小于HCC,但本研究中大部分HCA病灶表现为血流不丰富。可能原因:彩色多普勒对大的或者稍高速的血流敏感,对小的血流敏感度不高,而HCA中以静脉血流及低速低阻动脉为主,所以普通彩色多普勒图上常显示血流信号不丰富。然而,超声造影常显示明显的动脉期快速高增强,这与
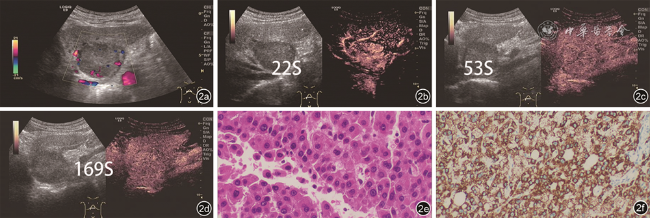
表2中的部分结果是相符的。既往研究报道,由于影像学特征存在重叠,HCA常误诊为CAML或HCC等其他肝内占位性病变
[2]。在本研究中80% HCA动脉期高增强,门静脉期或延迟期表现为等或低增强,即以“高-等-等”或“高-等-低”增强模式表现常见,甲胎蛋白阴性,不伴乙型肝炎或肝硬化等表现,与其他文献报道
[16]是相符的。而既往文献
[15, 16]中指出,动脉期向心性高增强是HCA的典型表现,常为均匀性高增强(除外出血的病灶),而在本研究中观察到HCA动脉期向心性高增强所占比例并不高,本研究中没有单独将其列为一个鉴别诊断要点,可能是因为本研究的病例数相对较少。HCC(AFP-)组的超声造影常表现为动脉期高增强,没有明显的向心性或离心性增强,常表现为典型的“高-低-低”增强模式。本研究显示,HCA病灶消退为等增强和低增强的时间均晚于HCC(AFP-)病灶(
P均<0.05),HCA病灶常在延迟期才消退为低增强,而HCC(AFP-)病灶造影剂消退较快,可发生在门静脉期早期甚至是动脉期后期消退为低增强,结合肝炎背景,由此可鉴别HCA与HCC(AFP-)。CAML的超声造影表现与HCA病灶相比差异没有统计学意义。CAML动脉期均匀高增强,门静脉及延迟期表现为高或等增强,其消退时间一般较长,常表现为“高-高-等”或“高-高-高”增强模式
[4]。但由于CAML中常含有脂肪成分,其二维超声表现以高回声为主。CAML、HCC(AFP-)及HCA的超声造影表现在诊断中具有一定的价值,但日常工作中仍然需要结合临床特点、常规二维超声表现进行鉴别诊断。