资料与方法
一、对象
二、仪器与方法
结果
一、超声表现
表1 9例MCSC的超声表现与病理结果 |
| 病例序号 | 年龄(岁) | 肿瘤位置 | 肿瘤最大径线(cm) | 内部回声 | Adler血流分级 | 后方回声增强 | 钙化 | BI-RADS分级 | 腋下淋巴结肿大 | 病理分型 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | 右乳、外上 | 4.12 | 实质性 | Ⅲ | 有 | 有 | 5 | 有 | 混合型化生性癌 |
| 2 | 57 | 左乳、外上 | 4.04 | 囊实性 | Ⅲ | 有 | 无 | 4c | 有 | SCC |
| 3 | 47 | 右乳、外上 | 2.78 | 实质性 | I | 有 | 有 | 5 | 无 | ASC |
| 4 | 47 | 右乳内侧 | 1.07 | 实质性 | Ⅲ | 有 | 无 | 4b | 无 | SCC |
| 5 | 44 | 右乳 | 15.00 | 囊实性 | Ⅲ | 有 | 无 | 5 | 有 | 混合型化生性癌 |
| 6 | 45 | 左乳、外上 | 2.74 | 实质性 | Ⅲ | 有 | 无 | 4c | 有 | 混合型化生性癌 |
| 7 | 79 | 右乳、外上 | 3.54 | 囊实性 | Ⅲ | 有 | 有 | 5 | 无 | 混合型化生性癌 |
| 8 | 46 | 右乳、外上 | 2.95 | 实质性 | Ⅲ | 有 | 无 | 5 | 无 | SCC |
| 9 | 51 | 左乳、外上 | 4.09 | 囊实性 | Ⅲ | 有 | 无 | 4c | 无 | 伴间叶分化的化生性癌 |
注:MCSC为含有鳞状细胞癌成分的乳腺化生性癌;SCC为单纯性鳞状细胞癌;ASC为腺鳞癌;BI-RADS为乳腺影像报告与数据系统 |
二、病理结果
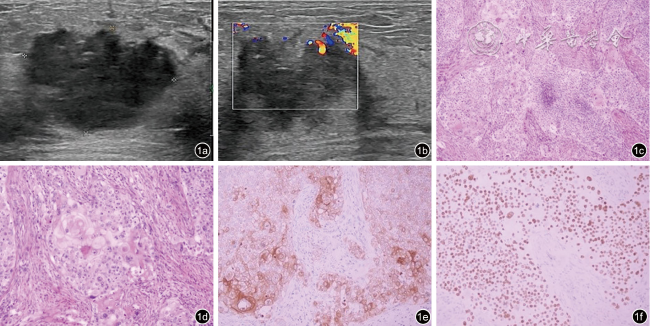
图1 含鳞状细胞癌成分的乳腺化生性癌的超声及病理图像(病例8)。图a为超声图像示肿块呈低回声,边界模糊形态不规则,未显示明显钙化;图b为彩色多普勒图像示肿块血流信号增多;图c为病理图像示肿瘤呈实性片状伴局部坏死,无腺管形成,可见角化珠和细胞间桥(HE ×100);图d为病理图像示肿瘤细胞核深染,核浆比增大,胞浆嗜酸,核分裂象可见(HE ×200);图e为免疫组织化学图像示肿瘤细胞CK5/6胞质阳性(EnVision法×200);图f为免疫组织化学图像示肿瘤细胞P40胞核弥漫强阳性(EnVision法 ×200) |
表2 9例MCSC的病理分型与腋窝淋巴结转移情况 |
| 病例序号 | 年龄(岁) | 病理分型 | 腋窝淋巴结转移 | 免疫组化 |
|---|---|---|---|---|
| 1 | 51 | 混合型化生性癌 | 腺癌(4/19枚) | ER(-)PR(-)Her-2(浸润性导管癌部分3+,鳞癌部分1+) |
| 2 | 57 | SCC | 无 | ER(10%,2+)PR(2%,2+)Her-2(-) |
| 3 | 47 | ASC | 无 | ER(1+,1%)PR(-)Her-2(-) |
| 4 | 47 | SCC | 无 | ER(-)PR(-)Her-2(2+) |
| 5 | 44 | 混合型化生性癌 | 鳞状细胞癌(1/14枚),腺癌(10/14枚) | ER(5%,2+)PR(2%,1+)Her-2(3+) |
| 6 | 45 | 混合型化生性癌 | 腺癌(2/21枚) | ER(-)PR(-)Her-2(-) |
| 7 | 79 | 混合型化生性癌 | 无 | ER(-)PR(-)Her-2(-) |
| 8 | 46 | SCC | 无 | ER(-)PR(-)Her-2(-) |
| 9 | 51 | 伴间叶分化的化生性癌 | 无 | ER(-)PR(-)Her-2(1+) |
注:MCSC为含有鳞状细胞癌成分的乳腺化生性癌;SCC为单纯性鳞状细胞癌;ASC为腺鳞癌;ER为雌激素受体;PR为孕激素受体;Her-2为人表皮生长因子受体-2 |