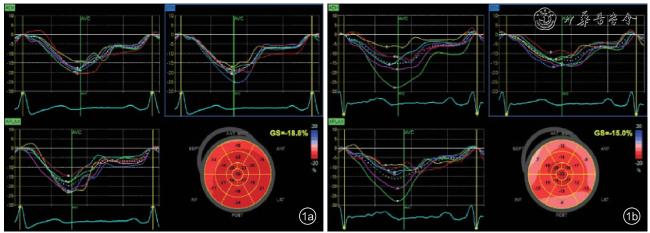
尽管存在争议,但是超声心动图应变参数在CA中的诊断作用是毋庸置疑的,之前的研究多数是针对心肌肥厚或者健康人群等,而本研究对这些参数在MM患者人群中的作用做出了新的探索。美国心脏核医学学会牵头发表的关于CA多模态诊断的专家共识中推荐RWT可作为诊断CA的指标之一,其诊断阈值为>0.42
[16],本研究结果显示,病例组RWT明显高于对照组(P<0.001),且MM人群中RWT预测是否合并CA的ROC曲线下面积为0.754,其具有较高的特异度(95%),诊断截断值为0.46,与既往研究结果相似。早期研究表明,GLS不仅可以用于评估CA的预后
[12],而且GLS降低结合应变图的相对“心尖保留”成像模式有助于诊断未分化的左心室肥厚,预测CA的敏感度及特异度分别达93%和82%
[7]。随后,相对区域应变比(relative regional strain ratio,RRSR)作为“心尖保留”的定量指标被提出,可以作为CA的有效评价指标
[8,13];SAB与RRSR的意义相似,其截断值为>2.9,敏感度为0.67,特异度为0.77
[14]。此外,Pagourelias等
[17]提出EFSR也可以在心肌肥厚患者中筛查CA,该指数>4.1时显示出良好的CA鉴别能力,其ROC曲线下面积达0.95,敏感度优于RRSR指标。本研究回顾性分析了2组的应变参数,结果显示病例组GLS明显降低,ASR及SAB均较对照组更高,提示病例组更倾向于“心尖保留”的成像特点。既往研究中以RRSR>1为截断值
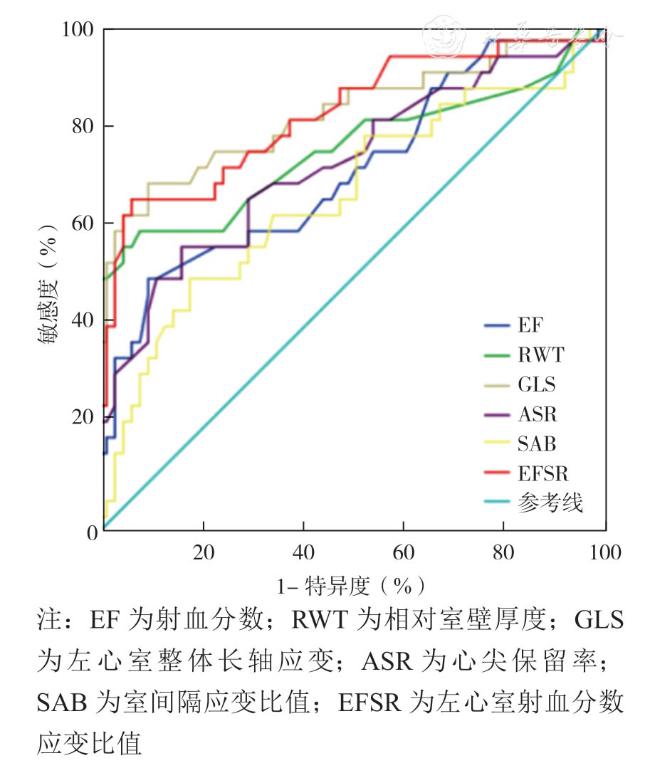
[13],但是在本研究中病例组ASR>1的比例较低,仅16%(5例),而且ASR诊断CA的截断值为0.72,与既往研究不相符,分析原因可能是MM作为特殊人群,与整体人群相比,发生CA的概率较高,导致临床医师会主动加强对这类患者的临床随访(如本中心对MM患者会常规进行斑点追踪超声心动图检查),更多的患者能够在CA的早期得到诊断,中晚期CA患者占比相对减少,从而使得“心尖保留”成像特点有减弱,CA截断值减小。此外,本研究中也引用了EFSR这个指标,结果显示病例组不仅有更高的EFSR (P<0.001),而且在诊断效能上优于ASR(ROC曲线下面积 0.82 vs 0.72),且具有较高的敏感度及特异度(65.6% vs 56.3%,93.4% vs 83.6%),与既往研究结果一致。通过单因素Logistic回归分析和ROC曲线评估,本研究发现LVEF、室壁厚度、RWT以及GLS、ASR、SAB、EFSR等参数在MM合并CA的诊断中均表现出一定的诊断效能。其中,GLS和EFSR的诊断效能尤为突出,ROC曲线下面积分别达到0.824和0.821。尽管ASR和SAB的效能略低(分别为0.724和0.661),但在评估心尖和室间隔的应变特征时仍提供了重要的补充信息。临床上常以生物标记物血清脑钠肽作为评估患者心肌损害的指标,本研究将血清脑钠肽与病例组超声心动图应变参数GLS、ASR、SAB及EFSR进行相关性分析,显示血清脑钠肽与GLS、ASR及EFSR呈正相关,提示应变的相关参数可以反映病例组的心脏功能受损严重程度,即心脏功能受损越重,GLS应变绝对值越低,ASR、SAB及EFSR值越高。
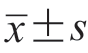 表示,组间比较采用单因素方差分析;不符合正态分布的计量资料以M(P25,P75)表示,组间比较采用非参数检验。计数资料以例(%)表示。采用单因素Logistic回归分析MM与MM合并CA存在差异的超声心动图参数。绘制ROC曲线分析超声心动图参数对MM合并CA的诊断效能。超声心动图参数与血清脑钠肽之间的相关性分析采用Pearson分析。以P<0.05为差异有统计学意义。
表示,组间比较采用单因素方差分析;不符合正态分布的计量资料以M(P25,P75)表示,组间比较采用非参数检验。计数资料以例(%)表示。采用单因素Logistic回归分析MM与MM合并CA存在差异的超声心动图参数。绘制ROC曲线分析超声心动图参数对MM合并CA的诊断效能。超声心动图参数与血清脑钠肽之间的相关性分析采用Pearson分析。以P<0.05为差异有统计学意义。