

2025 , Vol. 22 >Issue 04: 311 - 320
DOI: https://doi.org/10.3877/cma.j.issn.1672-6448.2025.04.006
卵巢颗粒细胞瘤与卵泡膜-纤维瘤组的超声特征及临床病理分析
Copy editor: 汪荣
收稿日期: 2025-03-06
网络出版日期: 2025-06-09
基金资助
广州市科技计划项目(2025A03J4286)
版权
Sonographic and clinicopathological characteristics of ovarian granulosa cell tumors and thecoma-fibroma group of tumors
Received date: 2025-03-06
Online published: 2025-06-09
Copyright
目的
对比分析卵巢颗粒细胞瘤(GCT)与卵泡膜-纤维瘤组(OTFG)肿瘤的超声及临床病理特征。
方法
回顾性纳入2015 年1 月至2024 年9 月于广东省妇幼保健院经手术病理证实的GCT(42 例)与OTFG(94 例)患者,比较分析其临床表现、超声特征及实验室指标。绘制ROC曲线分析抗苗勒管激素(AMH)诊断GCT 的敏感度、特异度和曲线下面积(AUC)。采用Cochran-Armitage 趋势检验分析肿瘤直径与盆腹腔积液及糖类抗原125(CA125)的相关变化趋势。
结果
GCT 组42 例中成人型颗粒细胞瘤36 例(36/42,85.71%),幼年型颗粒细胞瘤6 例(6/42,14.29%);OTFG 组94 例中卵泡膜纤维瘤59 例(59/94,62.77%),纤维瘤32 例(32/94,34.04%),卵泡膜细胞瘤3 例(3/94,3.19%)。OTFG 组以实性低回声为主(85/94,90.43%),后方回声衰减(81/94,86.17%),血流分级多为1 ~2 级(89/94,94.68%);GCT 组以囊实性(24/42,57.14%)及不均质实性回声(15/42,35.71%)为主,后方无衰减,血流分级多为3 ~4 级(37/42,88.09%)。OTFG 组盆腹腔积液及CA125 升高的发生率随肿瘤直径增加呈递增趋势(P<0.001),3 例合并Meigs 综合征的肿瘤直径均>10 cm 且伴有CA125 升高。而GCT 组仅CA125 升高的发生率随肿瘤直径增加呈递增趋势(P<0.05)。与OTFG 组相比,GCT 组的临床症状(83.33%,35/42 vs 57.45%,54/94)、性激素异常(50.00%,20/40 vs 12.20%,10/82)及AMH 升高发生率(80.00%,8/10 vs 0,0/24)均显著增加(P=0.003、<0.001、<0.001)。AMH 诊断GCT 的敏感度、特异度和ROC 曲线下面积分别为0.80、1.00 和0.90(P<0.001)。
结论
OTFG 与GCT 的超声表现、临床特征及实验室指标存在差异,超声检查结合AMH 等实验室检测及临床信息,可为卵巢性索间质肿瘤的术前诊断提供可靠依据。
田湘英 , 缪华章 , 徐玲 , 涂艳萍 , 张婕 , 相光华 , 赵贤哲 , 尚宁 . 卵巢颗粒细胞瘤与卵泡膜-纤维瘤组的超声特征及临床病理分析[J]. 中华医学超声杂志(电子版), 2025 , 22(04) : 311 -320 . DOI: 10.3877/cma.j.issn.1672-6448.2025.04.006
Objective
To compare the sonographic and clinicopathological characteristics of ovarian granulosa cell tumors (GCTs) and ovarian thecoma-fibroma group (OTFG) of tumors.
Methods
A retrospective analysis was conducted on 136 patients (42 GCTs and 94 OTFG tumors) with pathologically confirmed diagnoses between January 2015 and September 2024 from Guangdong Women and Children Hospital.Clinical manifestations, sonographic features, and laboratory indicators were compared.Receiver operating characteristic curve was plotted to analyze the sensitivity, specificity, and area under the curve (AUC) of anti-Müllerian hormone(AMH) in diagnosing GCT.Cochran Armitage trend test was used to analyze the correlation between tumor diameter, pelvic and peritoneal fluid accumulation, and carbohydrate antigen 125 (CA125).
Results
The GCT group comprised 36 adult-type GCTs (85.71%, 36/42) and 6 juvenile-type GCTs (14.29%, 6/42).The OTFG group included 59 fibrothecomas (62.77%, 59/94), 32 fibromas (34.04%, 32/94), and 3 thecomas (3.19%, 3/94).OTFG tumors predominantly presented as solid hypoechoic masses (90.43%, 85/94) with posterior acoustic attenuation(86.17%, 81/94) and low vascularity (grades 1-2: 94.68%, 89/94).In contrast, GCTs mainly manifested as cysticsolid (57.14%, 24/42) or heterogeneous solid masses (35.71%, 15/42) without posterior attenuation, with abundant vascularity (grades 3-4: 88.09%, 37/42).In the OTFG group, tumor diameter showed a significant positive correlation with ascites and elevated CA125 levels (P<0.001); all three cases with Meigs syndrome had tumors >10 cm and elevated CA125.In the GCT group, tumor diameter was only associated with elevated CA125 (P<0.05).The incidences of clinical symptoms (83.33% vs 57.45%), hormonal abnormalities (50.00% vs 12.20%), and elevated AMH levels(80.00% vs 0) were significantly higher in the GCT group compared to the OTFG group (P=0.003, <0.001, and <0.001,respectively).AMH demonstrated a sensitivity of 0.80, specificity of 1.00, and an AUC of 0.90 for diagnosing GCT(P<0.001).
Conclusion
OTFG tumors and GCTs exhibit distinct sonographic, clinical, and laboratory characteristics.Ultrasonography combined with AMH testing provides a reliable preoperative diagnostic framework for ovarian sex cord-stromal tumors.
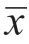 ±s 表示;计数资料采用例(%)表示,组间比较采用Fisher 精确概率法。绘制ROC 曲线分析AMH 诊断GCT 的敏感度、特异度和曲线下面积(area under curve,AUC)。采用Cochran-Armitage趋势检验分析肿瘤直径与盆腹腔积液及CA125 的相关变化趋势。单组率的点估计和95%置信区间采用Clopper-Pearson 精确法。以P<0.05 为差异具有统计学意义。
±s 表示;计数资料采用例(%)表示,组间比较采用Fisher 精确概率法。绘制ROC 曲线分析AMH 诊断GCT 的敏感度、特异度和曲线下面积(area under curve,AUC)。采用Cochran-Armitage趋势检验分析肿瘤直径与盆腹腔积液及CA125 的相关变化趋势。单组率的点估计和95%置信区间采用Clopper-Pearson 精确法。以P<0.05 为差异具有统计学意义。表1 OTFG 组与GCT 组的临床与超声特征比较[例(%)] |
| 临床与超声特征 | 合计(n=136) | OTFG 组(n=94) | GCT 组(n=42) | P值 |
|---|---|---|---|---|
| 年龄 | 0.344 | |||
| < 30 岁 | 26(19.12) | 19(20.21) | 7(16.67) | |
| 30 ~ 50 岁 | 68(50.00) | 43(45.74) | 25(59.52) | |
| > 50 岁 | 42(30.88) | 32(34.04) | 10(23.81) | |
| 出现临床症状 | 89(65.44) | 54(57.45) | 35(83.33) | 0.003 |
| 合并子宫内膜病变 | 28(28.28) | 20(29.85) | 8(25.00) | 0.779 |
| 肿瘤标志物指标异常 | 37(27.82) | 25(26.88) | 12(30.00) | 0.833 |
| 性激素指标异常 | 30(24.59) | 10(12.20) | 20(50.00) | < 0.001 |
| AMH 指标异常 | 8(25.00) | 0(0) | 8(80.00) | < 0.001 |
| 肿块直径 | 0.059 | |||
| < 5 cm | 52(38.24) | 42(44.68) | 10(23.81) | |
| 5 ~ 10 cm | 57(41.91) | 36(38.30) | 21(50.00) | |
| > 10 cm | 27(19.85) | 16(17.02) | 11(26.19) | |
| 肿块位置 | 0.754 | |||
| 左侧 | 60(44.12) | 41(43.62) | 19(45.24) | |
| 右侧 | 73(53.68) | 50(53.19) | 23(54.76) | |
| 双侧 | 3(2.21) | 3(3.19) | 0(0) | |
| 内部回声 | < 0.001 | |||
| 囊性 | 5(3.68) | 2(2.13) | 3(7.14) | |
| 实性 | 100(73.53) | 85(90.43) | 15(35.71) | |
| 囊实性 | 31(22.79) | 7(7.45) | 24(57.14) | |
| 形态 | < 0.001 | |||
| 规则 | 116(85.29) | 88(93.62) | 28(66.67) | |
| 不规则 | 20(14.71) | 6(6.38) | 14(33.33) | |
| 肿瘤内部囊性变 | 48(35.29) | 17(18.09) | 31(73.81) | < 0.001 |
| 后方回声衰减 | 81(59.56) | 81(86.17) | 0(0) | < 0.001 |
| 血流等级 | < 0.001 | |||
| 1 | 28(20.59) | 28(29.79) | 0(0) | |
| 2 | 66(48.53) | 61(64.89) | 5(11.90) | |
| 3 | 29(21.32) | 5(5.32) | 24(57.14) | |
| 4 | 13(9.56) | 0(0) | 13(30.95) | |
| 肿瘤自发破裂 | 6(4.41) | 1(1.06) | 5(11.90) | 0.011 |
注:OTFG 为卵泡膜-纤维瘤组;GCT 为颗粒细胞瘤 |
图2 患者,50 岁,卵巢纤维瘤误诊为子宫肌瘤超声及病理图像。图a 为超声图像示子宫右旁低回声包块,大小69 mm×40 mm,后伴栅栏状衰减,与子宫右侧壁关系密切,未探及子宫来源血供,血流等级2 级,超声误诊为子宫肌瘤;图b 为病理图像见梭形细胞呈编织状排列,诊断为右卵巢纤维瘤 |
图3 患者,47 岁,卵巢卵泡膜细胞瘤误诊为上皮源性肿瘤超声及病理图像。图a 为超声图像示子宫左上方囊性包块,大小147 mm×128 mm,内见多发分隔,部分囊内见均匀细密点状回声,血流等级2 级,超声误诊为上皮源性肿瘤;图b 为病理图像见梭形细胞组成,伴钙化、出血及囊性变,诊断为左卵巢卵泡膜细胞瘤 |
图4 患者,51 岁,卵巢成人型颗粒细胞瘤误诊为上皮源性肿瘤超声及病理图像。图a 为超声图像示右卵巢囊实性包块,大小97 mm×44 mm,血流等级3 ~4 级,超声误诊为上皮源性肿瘤;图b 为病理图像见肿瘤细胞呈灶状或片状分布,细胞圆形及卵圆形,诊断为成人型颗粒细胞瘤 |
图6 患者,38 岁,卵巢纤维瘤超声及病理图像。图a 为超声图像示右卵巢实性低回声,大小33 mm×19 mm,后方回声衰减明显,血流等级1级,超声诊断纤维瘤;图b 为病理图像见肿瘤由纤维细胞和成纤维细胞构成,呈编织状排列,诊断为纤维瘤 |
图7 患者,51 岁,卵泡膜纤维瘤超声及病理图像。图a 为超声图像示左卵巢实性低回声,大小85 mm×58 mm,后伴栅栏状衰减,血流等级2级,超声诊断性索间质来源肿瘤;图b 为病理图像见卵泡膜细胞与纤维成分交错分布,呈编织状排列,诊断为卵泡膜纤维瘤 |
图9 患者,11 岁,幼年型颗粒细胞瘤超声及病理图像。图a 为超声图像示左卵巢实性低回声,内见高回声纤维分隔呈网格状(白色箭头),大小118 mm×60 mm,血流等级3 ~4 级,超声诊断性索间质来源肿瘤;图b 为病理图像见肿瘤细胞呈巢状分布,诊断为幼年型颗粒细胞瘤 |
图10 患者,33 岁,成年型颗粒细胞瘤超声及病理图像(超声未定性)。图a 为超声图像示右卵巢囊实性包块,囊性为主,大小92 mm×61 mm,内见蜂窝状无回声,血流等级3 ~4 级,超声未定性;图b 为病理图像见肿瘤细胞呈弥漫片状生长,细胞呈卵圆形,诊断为成年型颗粒细胞瘤 |
| 1 |
Ray-Coquard I, Morice P, Lorusso D, et al.Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up [J].Ann Oncol, 2018, 29(Suppl 4): 1-18.
|
| 2 |
Haroon S, Zia A, Idrees R, et al.Clinicopathological spectrum of ovarian sex cord-stromal tumors: a 20-year retrospective study in a developing country [J].J Ovarian Res, 2013, 6: 85.
|
| 3 |
WHO Classification of Tumours Editorial Board.Female genital tumours [M].5th ed.Lyon: International Agency for Research on Cancer, 2020: 93-111.
|
| 4 |
中国抗癌协会妇科肿瘤专业委员会.卵巢恶性肿瘤诊断与治疗指南(2021 年版)[J].中国癌症杂志, 2021, 31(6): 490-500.
|
| 5 |
Young RH.Ovarian sex cord-stromal tumours and their mimics [J].Pathology, 2018, 50(1): 5-15.
|
| 6 |
Andreotti RF, Timmerman D, Strachowski LM, et al.O-RADS US risk stratification and management system: a consensus guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee [J].Radiology, 2020, 294(1): 168-185.
|
| 7 |
Timmerman D, Valentin L, Bourne TH, et al.Terms, definitions and measurements to describe the sonographic features of adnexal tumors:a consensus opinion from the International Ovarian Tumor Analysis(IOTA) group [J].Ultrasound Obstet Gynecol, 2002, 16(5): 500-505.
|
| 8 |
Prat J.Abridged republication of FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum [J].Cancer, 2015,121(19): 3452-3454.
|
| 9 |
Chen H, Liu Y, Shen LF, et al.Ovarian thecoma-fibroma groups:clinical and sonographic features with pathological comparison [J].J Ovarian Res, 2016, 9(1): 81.
|
| 10 |
Hillman RT, Celestino J, Terranova C, et al.KMT2D/MLL2 inactivation is associated with recurrence in adult-type granulosa cell tumors of the ovary [J].Nat Commun, 2018, 9(1): 3470.
|
| 11 |
肖欢, 唐毅, 王荞, 等.儿童卵巢幼年颗粒细胞瘤超声表现及病理对照研究[J/CD].中华医学超声杂志(电子版), 2021, 18(10): 985-990.
|
| 12 |
Haltia UM, Hallamaa M, Tapper J, et al.Roles of human epididymis protein 4, carbohydrate antigen 125, inhibin B and anti-Mullerian hormone in the differential diagnosis and follow-up of ovarian granulosa cell tumors [J].Gynecol Oncol, 2017, 144(1): 83-89.
|
| 13 |
Colombo N, Parma G, Zanagnolo V, et al.Management of ovarian stromal cell tumors [J].J Clin Oncol, 2007, 25(20): 2944-2951.
|
| 14 |
Jamieson S, Fuller PJ.Molecular pathogenesis of granulosa cell tumors of the ovary [J].Endocr Rev, 2012, 33(1): 109-144.
|
| 15 |
Plett H, Ricciardi E, Vacaru V, et al.Adult ovarian granulosa cell tumors: analysis of outcomes and risk factors for recurrence [J].Int J Gynecol Cancer, 2023, 33(5): 734-740.
|
| 16 |
Petrone M, Bergamini A, Tateo S, et al.Transvaginal ultrasound in evaluation and follow-up of ovarian granulosa cell tumors [J].Int J Gynecol Cancer, 2020, 30(9): 1384-1389.
|
/
| 〈 |
|
〉 |